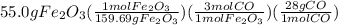Chemistry, 09.07.2019 10:30 isiahamccoy8822
What mass of co is needed to react completely with 55.0 g of fe2o3 in the reaction: fe2o3(s) + co(g) → fe(s) + co2(g)? 4.82 g co 9.64 g co 14.5 g co 28.9 g co

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
What mass of co is needed to react completely with 55.0 g of fe2o3 in the reaction: fe2o3(s) + co(g...
Questions

Mathematics, 29.10.2020 03:30

Computers and Technology, 29.10.2020 03:30

History, 29.10.2020 03:30


English, 29.10.2020 03:30

English, 29.10.2020 03:30

History, 29.10.2020 03:30


Biology, 29.10.2020 03:30

Mathematics, 29.10.2020 03:30

Engineering, 29.10.2020 03:30

Biology, 29.10.2020 03:30




Mathematics, 29.10.2020 03:30

Mathematics, 29.10.2020 03:30



English, 29.10.2020 03:30

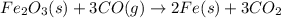
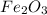 is 159.69 gram per mol and the molar mass of CO is 28 gram per mol. The calculations are shown below:
is 159.69 gram per mol and the molar mass of CO is 28 gram per mol. The calculations are shown below: