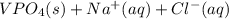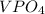Chemistry, 09.07.2019 08:00 aaronlikly
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rules to identify the composition of the salt that precipitates out of the solution. a. vpo4 b. na3po4 c. vcl3 d. nacl

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 13:30
Elaborate on the reason(s) that matter is said to move even as in a solid state. select one: a. the particles are bound through intermolecular forces but are able to move past each other with relative freedom. b. the particles have sufficient energy to become an ionized gas and are in the most common state of matter in the universe. c. the particles are not able to move out of their positions relative to one another, but do have small vibrational movements. d. the particles are not bound to one another, move quickly, have a low density, and are able to spread apart from one another if unconstrained.
Answers: 1
You know the right answer?
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rule...
Questions

History, 28.09.2019 01:00


Mathematics, 28.09.2019 01:00




History, 28.09.2019 01:00

Mathematics, 28.09.2019 01:00


Mathematics, 28.09.2019 01:00




Biology, 28.09.2019 01:00

History, 28.09.2019 01:00


English, 28.09.2019 01:00

Mathematics, 28.09.2019 01:00


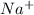 ,
,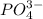 ,
,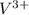 , and
, and 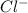 . The products formed in the reaction would be NaCl and VPO
. The products formed in the reaction would be NaCl and VPO . As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with
. As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with 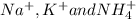 .
.