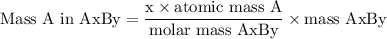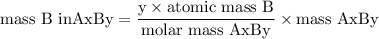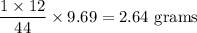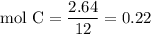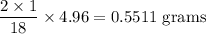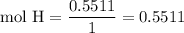Chemistry, 09.07.2019 07:30 kitttimothy55
Complete combustion of 3.20g of a hydrocarbon produced 9.69g of co2 and 4.96g of h2o. what is the empirical formula for the hydrocarbon?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Complete combustion of 3.20g of a hydrocarbon produced 9.69g of co2 and 4.96g of h2o. what is the em...
Questions

Mathematics, 08.07.2019 01:00


Mathematics, 08.07.2019 01:00


Physics, 08.07.2019 01:00


Mathematics, 08.07.2019 01:00





History, 08.07.2019 01:00


Mathematics, 08.07.2019 01:00

Business, 08.07.2019 01:00


Mathematics, 08.07.2019 01:00

Mathematics, 08.07.2019 01:00

Mathematics, 08.07.2019 01:00