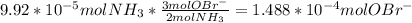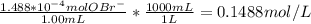Chemistry, 09.07.2019 04:30 wedderman6049
Ammonia reacts with hypobromite, obr, by the reaction 2nh3 3obr n n2 3br 3h2o. what is the molarity of a hypobromite solution if 1.00 ml of the obr solution reacts with 1.69 mg of nh3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1


You know the right answer?
Ammonia reacts with hypobromite, obr, by the reaction 2nh3 3obr n n2 3br 3h2o. what is the molarity...
Questions

Advanced Placement (AP), 18.04.2021 18:00

History, 18.04.2021 18:00



Mathematics, 18.04.2021 18:00

Mathematics, 18.04.2021 18:00

Mathematics, 18.04.2021 18:00

English, 18.04.2021 18:00

English, 18.04.2021 18:00


Arts, 18.04.2021 18:00

English, 18.04.2021 18:00

Social Studies, 18.04.2021 18:00

Business, 18.04.2021 18:00

Mathematics, 18.04.2021 18:00

English, 18.04.2021 18:00

English, 18.04.2021 18:00

Chemistry, 18.04.2021 18:00


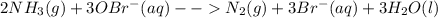
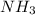 =1.69mg
=1.69mg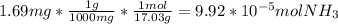
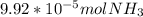 :
: