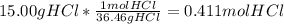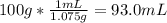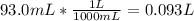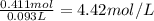Chemistry, 09.07.2019 04:00 shelby7629
The mass percentage of hydrochloric acid within a solution is 15.00%. given that the density of this solution is 1.075 g/ml, find the molarity of the solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
The mass percentage of hydrochloric acid within a solution is 15.00%. given that the density of this...
Questions

Mathematics, 05.05.2021 03:10

Computers and Technology, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10

English, 05.05.2021 03:10




Mathematics, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10

English, 05.05.2021 03:10




Mathematics, 05.05.2021 03:10


Computers and Technology, 05.05.2021 03:10

Social Studies, 05.05.2021 03:10


History, 05.05.2021 03:10

Arts, 05.05.2021 03:10