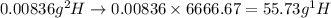Chemistry, 18.11.2019 04:31 robert7248
Deuterium, 2h (2.0140 u), is sometimes used to replace the principal hydrogen isotope 1h in chemical studies. the percent natural abundance of deuterium is 0.015%. part a if it can be done with 100% efficiency, what mass of naturally occurring hydrogen gas would have to be processed to obtain a sample containing 2.50ã1021 2h atoms?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Deuterium, 2h (2.0140 u), is sometimes used to replace the principal hydrogen isotope 1h in chemical...
Questions

History, 07.12.2020 19:20



English, 07.12.2020 19:20



Mathematics, 07.12.2020 19:20


Business, 07.12.2020 19:20


Mathematics, 07.12.2020 19:20


Chemistry, 07.12.2020 19:20

Mathematics, 07.12.2020 19:20


Chemistry, 07.12.2020 19:20

Advanced Placement (AP), 07.12.2020 19:20



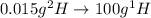
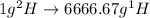
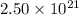 .
. 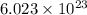 atoms thus,
atoms thus,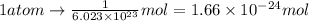
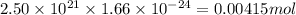
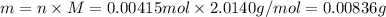
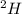 is 0.00836 g
is 0.00836 g