Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Sodium hydroxide reacts with carbon dioxide to form sodium carbonate and water: 2 naoh(s) + co2(g)...
Questions


Mathematics, 14.12.2021 02:20

Mathematics, 14.12.2021 02:20

Mathematics, 14.12.2021 02:20


Chemistry, 14.12.2021 02:20


Mathematics, 14.12.2021 02:20


Biology, 14.12.2021 02:20


Mathematics, 14.12.2021 02:20

SAT, 14.12.2021 02:20



Biology, 14.12.2021 02:20




Mathematics, 14.12.2021 02:20

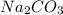 prepared can be 3.18 grams.
prepared can be 3.18 grams.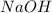 .
.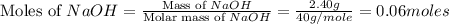
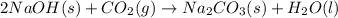
 of
of