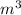Chemistry, 06.10.2019 12:00 biancaadenisee2
The equilibrium fraction of lattice sites that are vacant in silver (ag) at 500â°c is 0.5 ã 10-6. calculate the number of vacancies (per meter cubed) at 500â°c. assume a density of 10.35 g/cm3 for ag, and note that aag = 107.87 g/mol.

Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 13:30
Where are electrons with the lowest energy found? in the nucleus farthest from the nucleus outside the atom closest to the nucleus
Answers: 1
You know the right answer?
The equilibrium fraction of lattice sites that are vacant in silver (ag) at 500â°c is 0.5 ã 10-6. ca...
Questions

English, 17.11.2020 21:40


English, 17.11.2020 21:40


Social Studies, 17.11.2020 21:40


Biology, 17.11.2020 21:40



Chemistry, 17.11.2020 21:40


Mathematics, 17.11.2020 21:40

Biology, 17.11.2020 21:40


Mathematics, 17.11.2020 21:40

Business, 17.11.2020 21:40

Chemistry, 17.11.2020 21:40



Mathematics, 17.11.2020 21:40

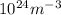
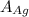 = 107.87 g/mol
= 107.87 g/mol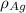 = 10.35
= 10.35 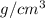 = 10.35
= 10.35 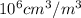 = 10.35 ×
= 10.35 × 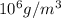
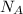 = 6.022 ×
= 6.022 × 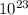 atoms/mol
atoms/mol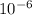
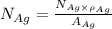
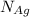 = Total number of lattice sites in Ag
= Total number of lattice sites in Ag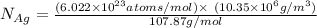
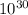 atoms/
atoms/