
Chemistry, 09.07.2019 00:30 deandrehudson18
Which variable determines the electron energy in the hydrogen atom according to bohr’s model

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
You know the right answer?
Which variable determines the electron energy in the hydrogen atom according to bohr’s model...
Questions

Physics, 16.01.2020 11:31


Mathematics, 16.01.2020 11:31


Mathematics, 16.01.2020 11:31

Mathematics, 16.01.2020 11:31



Chemistry, 16.01.2020 11:31

Mathematics, 16.01.2020 11:31


Mathematics, 16.01.2020 11:31

Social Studies, 16.01.2020 11:31

Social Studies, 16.01.2020 11:31





History, 16.01.2020 11:31

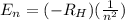
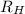 = Rydberg's constant
= Rydberg's constant

