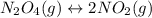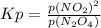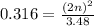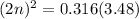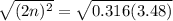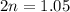Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
For the equilibrium, n2o4(g)< > 2no2(g), the equilibrium constant kp = 0.316. calculate the e...
Questions


English, 09.09.2019 22:30


Biology, 09.09.2019 22:30





English, 09.09.2019 22:30



Mathematics, 09.09.2019 22:30



Law, 09.09.2019 22:30

English, 09.09.2019 22:30




English, 09.09.2019 22:30

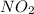 is 1.05 atm.
is 1.05 atm.