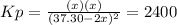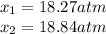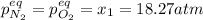Chemistry, 08.07.2019 23:30 juansebas35
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is present at a partial pressure of 37.30 atm, what will the partial pressures of n2 and o2 be at equilibrium? 1827 atm 38.08 atm 1.725 atm 36.55 atm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is pres...
Questions




Mathematics, 29.01.2021 16:50



Social Studies, 29.01.2021 16:50







Computers and Technology, 29.01.2021 16:50






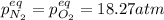
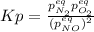
 , changes to:
, changes to: