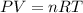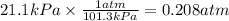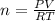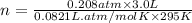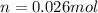Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Ahuman lung at maximum capacity has a volume of 3.0 liters. if the partial pressure of oxygen in the...
Questions

Mathematics, 24.08.2020 03:01



Mathematics, 24.08.2020 03:01

Physics, 24.08.2020 03:01

Biology, 24.08.2020 03:01

Health, 24.08.2020 03:01


Mathematics, 24.08.2020 03:01




History, 24.08.2020 03:01


Mathematics, 24.08.2020 03:01



Physics, 24.08.2020 03:01