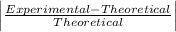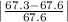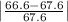Student a performed gravimetric analysis for sulfate in her unknown using the same procedures we did . results of her three trials were 68.6%, 66.2% and 67.1% sulfate. student b analyzed the same unknown his results were 66.7%, 66.6% and 66.5%. the unknown was sodium sulfate. calculate a percent e rror fo r student a and for student b using as the accepted value the theoretical value for sulfate in sodium sulfate based on molar mass . [y ou may use the internet, a textbook, the chemistry c ommunity learning center (cclc) or any other resource for an explanation/description of percent error .] which student , a or b, was more accurate? which student was more precise? explain your answers

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3
You know the right answer?
Student a performed gravimetric analysis for sulfate in her unknown using the same procedures we did...
Questions


Mathematics, 12.02.2022 05:10




Social Studies, 12.02.2022 05:10






Advanced Placement (AP), 12.02.2022 05:10



Advanced Placement (AP), 12.02.2022 05:20

Biology, 12.02.2022 05:20

Mathematics, 12.02.2022 05:20