
Chemistry, 08.07.2019 15:30 littlebrain2672
Calculate the concentrations of iron(iii) ions and scn- ions when 5.00 ml of 0.002m iron(iii) nitrate is mixed with 4.00 ml of 0.002 m nascn and dilute nitric acid is added to bring the total volume of the solution to 20.0 ml. what is the concentration of iron(iii) ions?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1
You know the right answer?
Calculate the concentrations of iron(iii) ions and scn- ions when 5.00 ml of 0.002m iron(iii) nitrat...
Questions

Mathematics, 08.12.2019 19:31


Health, 08.12.2019 19:31


English, 08.12.2019 19:31

Business, 08.12.2019 19:31

Mathematics, 08.12.2019 19:31

Mathematics, 08.12.2019 19:31

Physics, 08.12.2019 19:31




Geography, 08.12.2019 19:31

Mathematics, 08.12.2019 19:31

Social Studies, 08.12.2019 19:31

Mathematics, 08.12.2019 19:31


Mathematics, 08.12.2019 19:31

Biology, 08.12.2019 19:31

History, 08.12.2019 19:31

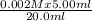 = 5 x 10⁻⁴
= 5 x 10⁻⁴

