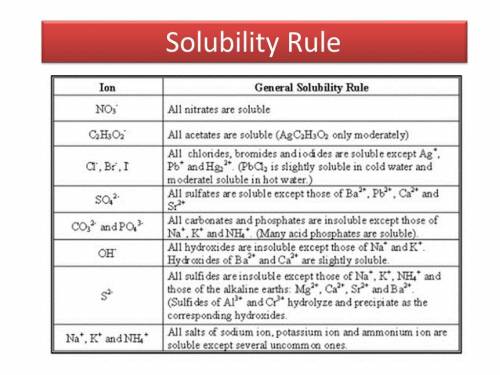
Chemistry, 16.12.2019 00:31 garciavergaraana
Asolution of zinc nitrate is added to a solution of magnesium sulfate. express your answer as a chemical equation. identify all of the phases in your answer. enter no reaction if no reaction occurs.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1

Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2
You know the right answer?
Asolution of zinc nitrate is added to a solution of magnesium sulfate. express your answer as a chem...
Questions






German, 23.11.2020 22:20


Mathematics, 23.11.2020 22:20



History, 23.11.2020 22:20

Mathematics, 23.11.2020 22:20




Mathematics, 23.11.2020 22:20

English, 23.11.2020 22:20


History, 23.11.2020 22:20