Chemistry, 08.07.2019 11:00 Brainly264
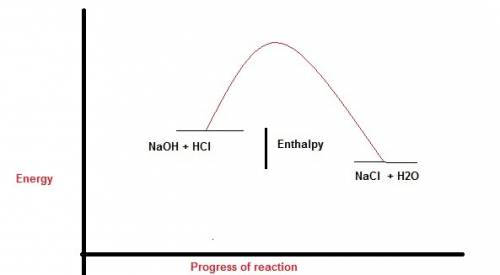
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-54 kj a. gambarlah diagram tingkat energi untuk reaksi tersebut b. berapakah perubahan entalpi jika 100 ml naoh 1 m ? c. berapakah perubahan entalpi jika 10 ml hcl 1 m direaksikan dengan 20 ml naoh 1 m ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-5...
Questions


Biology, 28.08.2019 17:00




Mathematics, 28.08.2019 17:00


Health, 28.08.2019 17:00

English, 28.08.2019 17:00


English, 28.08.2019 17:00

Mathematics, 28.08.2019 17:00


Mathematics, 28.08.2019 17:00

Mathematics, 28.08.2019 17:00

Mathematics, 28.08.2019 17:00

Chemistry, 28.08.2019 17:00