Chemistry, 08.07.2019 08:00 supergraciepie
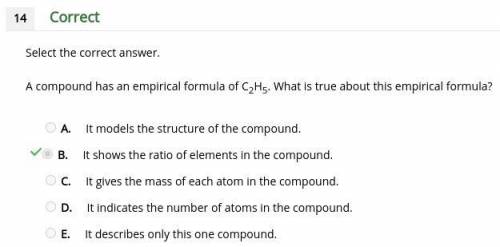
Acompound has an empirical formula of c2h5. what is true about this empirical formula? a. it models the structure of the compound. b. it shows the ratio of elements in the compound. c. it gives the mass of each atom in the compound. d. it indicates the number of atoms in the compound. e. it describes only this one compound.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Acompound has an empirical formula of c2h5. what is true about this empirical formula? a. it models...
Questions


History, 23.08.2019 21:20

History, 23.08.2019 21:20


English, 23.08.2019 21:20



Geography, 23.08.2019 21:20


Health, 23.08.2019 21:20

Biology, 23.08.2019 21:20


Mathematics, 23.08.2019 21:20

Health, 23.08.2019 21:20


Mathematics, 23.08.2019 21:20



Social Studies, 23.08.2019 21:20

Social Studies, 23.08.2019 21:20