
Chemistry, 08.07.2019 06:00 sotoamerica0814
In the atmosphere, under the effect of ultraviolet rays, oxygen molecules combine to form ozone molecules, and ozone molecules also photodissociate to form oxygen molecules. which of the following would be observed if this reaction is in dynamic equilibrium? the concentration of oxygen will increase. the concentration of ozone will decrease. the rates of the forward and reverse reactions will increase. the rates of the forward and reverse reactions will remain the same.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
In the atmosphere, under the effect of ultraviolet rays, oxygen molecules combine to form ozone mole...
Questions



Mathematics, 31.08.2021 21:50

Mathematics, 31.08.2021 21:50

Mathematics, 31.08.2021 21:50


Social Studies, 31.08.2021 21:50

Mathematics, 31.08.2021 21:50



Computers and Technology, 31.08.2021 21:50

Mathematics, 31.08.2021 21:50

English, 31.08.2021 21:50

Engineering, 31.08.2021 21:50



Mathematics, 31.08.2021 21:50


Mathematics, 31.08.2021 21:50

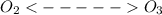 in presence of UV rays. Hence the rates of the forward and backward reactions remains the same in the atmosphere even after photo dissociation.
in presence of UV rays. Hence the rates of the forward and backward reactions remains the same in the atmosphere even after photo dissociation.

