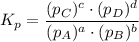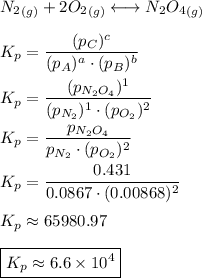Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Consider the equilibrium, n2(g) + 2o2(g) < > n2o4(g). calculate the equilibrium constant, kpi...
Questions

English, 14.10.2019 18:30

Biology, 14.10.2019 18:30

Chemistry, 14.10.2019 18:30


English, 14.10.2019 18:30


Mathematics, 14.10.2019 18:30


Mathematics, 14.10.2019 18:30



Mathematics, 14.10.2019 18:30







English, 14.10.2019 18:30