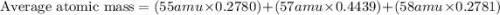Chemistry, 07.07.2019 23:00 a897coleman
An undiscovered element has three naturally occurring isotopes of x-55, x-57, and x-58. isotope x-55 has an abundance of 27.80 % and isotope x-57 has an abundance of 44.39 %. what is the average mass of this element in amu?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
An undiscovered element has three naturally occurring isotopes of x-55, x-57, and x-58. isotope x-55...
Questions







Mathematics, 03.04.2020 19:31






Mathematics, 03.04.2020 19:32




Mathematics, 03.04.2020 19:32

English, 03.04.2020 19:32


History, 03.04.2020 19:33

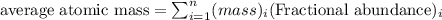 .....(1)
.....(1)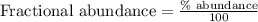
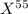 ,
,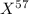 ,
,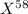 ,
,