
Chemistry, 07.07.2019 22:00 brittanyfox411
During a water treatment program, 127 grams of calcium nitrate, ca(no3)2, is dissolved in water. the final volume of the solution is 2,300 milliliters. what is the molarity of the solution? refer to the periodic table to you answer. express your answer to two significant figures. the molarity of the solution is m.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3


You know the right answer?
During a water treatment program, 127 grams of calcium nitrate, ca(no3)2, is dissolved in water. the...
Questions








Social Studies, 24.01.2020 21:31












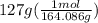
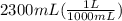
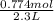 = 0.34 M
= 0.34 M


