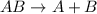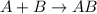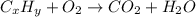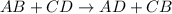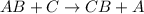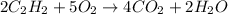Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
What reaction type is depicted by the following equation? 2c2h2 + 5o2 4co2 + 2h2o decomposition com...
Questions




Mathematics, 02.09.2019 17:00

Social Studies, 02.09.2019 17:00

Mathematics, 02.09.2019 17:00


Computers and Technology, 02.09.2019 17:00

English, 02.09.2019 17:00


Mathematics, 02.09.2019 17:00

Biology, 02.09.2019 17:00


Biology, 02.09.2019 17:00

Chemistry, 02.09.2019 17:00


English, 02.09.2019 17:00


Biology, 02.09.2019 17:00

Mathematics, 02.09.2019 17:00