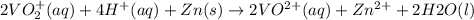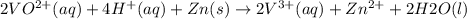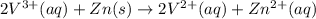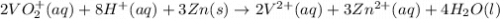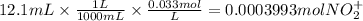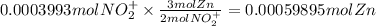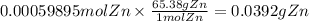Chemistry, 07.07.2019 19:30 tladitidimatso1783
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction from +5 to +4: 2 vo2+(aq) + 4 h +(aq) + zn(s) → 2 vo2+(aq) + zn2+(aq) + 2 h2o(l) reduction from +4 to +3: 2 vo2+(aq) + zn(s) + 4 h +(aq) → 2 v3+(aq) + zn2+(aq) + 2 h2o(l) reduction from +3 to +2: 2 v3+(aq) + zn(s) → 2 v2+(aq) + zn2+(aq) if you had 12.1 ml of a 0.0033 m solution of vo2+(aq), how many grams of zn metal would be required to completely reduce the vanadium?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

You know the right answer?
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction fro...
Questions


Mathematics, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00

English, 26.04.2021 14:00


World Languages, 26.04.2021 14:00



Mathematics, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00

World Languages, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00

French, 26.04.2021 14:00

Mathematics, 26.04.2021 14:00


Mathematics, 26.04.2021 14:00



Chemistry, 26.04.2021 14:00