Chemistry, 07.07.2019 18:30 cougar9754
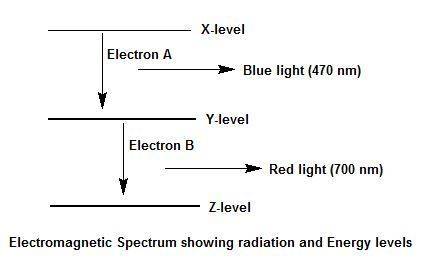
Electron a falls from energy level x to energy level y and releases blue light. electron b falls from energy level y to energy level z and releases red light. which transition, from x to y or from y to z, has a greater energy difference? explain your answer and how you arrived at it. use a diagram of the electromagnetic spectrum.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2



Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Electron a falls from energy level x to energy level y and releases blue light. electron b falls fro...
Questions

Mathematics, 04.07.2019 03:00

Mathematics, 04.07.2019 03:00


Mathematics, 04.07.2019 03:00


Mathematics, 04.07.2019 03:00


Mathematics, 04.07.2019 03:00

Biology, 04.07.2019 03:00




Physics, 04.07.2019 03:00

Mathematics, 04.07.2019 03:00

Health, 04.07.2019 03:00


English, 04.07.2019 03:00

History, 04.07.2019 03:00

Mathematics, 04.07.2019 03:00

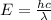
 = Wavelength of particle
= Wavelength of particle