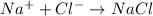It is difficult to break the ionic bonds in a compound because of a. good conductivity between ions. b. small distance between the ions. c. strong attraction between ions. ionic compounds are solid at room temperature because they a. high melting points b. ion with similar charges c. no valence electrons

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
It is difficult to break the ionic bonds in a compound because of a. good conductivity between ion...
Questions


History, 27.07.2019 22:30

History, 27.07.2019 22:30

Mathematics, 27.07.2019 22:30



Health, 27.07.2019 22:30

Social Studies, 27.07.2019 22:30



Computers and Technology, 27.07.2019 22:30


Advanced Placement (AP), 27.07.2019 22:30






Mathematics, 27.07.2019 22:30

Mathematics, 27.07.2019 22:30

![Na:11:[Ne]3s^1](/tpl/images/0062/6064/98162.png)
![Na^+:10:[Ne]](/tpl/images/0062/6064/a96fa.png)
![Cl:17:[Ne]3s^23p^5](/tpl/images/0062/6064/bf183.png)
![Cl^-:18:[Ne]3s^23p^6](/tpl/images/0062/6064/1914b.png)