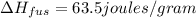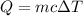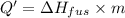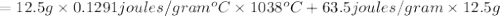Agoldsmith melts 12.4 grams of gold to make a ring. the temperature of the gold rises from 26°c to 1064°c, and then the gold melts completely. if gold’s specific heat is 0.1291 joules/gram degree celsius and its heat of fusion is 63.5 joules/gram, how much energy is gained by the gold? the gold gains a total of joules of energy. i was given this by somebody. no idea what to do with this. qj=(12.4g*(1064-26)°c*0.1291j/g/°c) +(12.4g*63.5j)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Agoldsmith melts 12.4 grams of gold to make a ring. the temperature of the gold rises from 26°c to 1...
Questions

English, 04.02.2020 15:53


Mathematics, 04.02.2020 15:53


Mathematics, 04.02.2020 15:53


Mathematics, 04.02.2020 15:53




Mathematics, 04.02.2020 15:53

Health, 04.02.2020 15:53

Mathematics, 04.02.2020 15:53


Biology, 04.02.2020 15:53


Mathematics, 04.02.2020 15:53


Mathematics, 04.02.2020 15:53