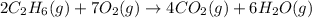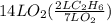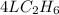Chemistry, 07.07.2019 15:00 villarrealc1987
2c2h6(g) + 7o2(g) → 4co2(g) + 6h2o(g) in the equation, if 14 l of ethane (c2h6) and 14 l of oxygen (o2) combined and burned to completion, which gas will be leftover after the reaction, and what is the volume of that gas remaining

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
2c2h6(g) + 7o2(g) → 4co2(g) + 6h2o(g) in the equation, if 14 l of ethane (c2h6) and 14 l of oxygen (...
Questions





Mathematics, 21.12.2019 17:31

English, 21.12.2019 17:31

Social Studies, 21.12.2019 17:31

Chemistry, 21.12.2019 17:31



Chemistry, 21.12.2019 17:31

Social Studies, 21.12.2019 17:31





Mathematics, 21.12.2019 17:31


English, 21.12.2019 17:31