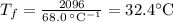Chemistry, 07.07.2019 04:30 IsabellaGracie
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g of water at 28.9 ◦c in an insulated container? the specific heat of lead is 0.128 j/g◦c.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g...
Questions

Mathematics, 07.12.2020 03:30


English, 07.12.2020 03:30



Mathematics, 07.12.2020 03:30

Mathematics, 07.12.2020 03:30

Mathematics, 07.12.2020 03:30

Mathematics, 07.12.2020 03:30



Mathematics, 07.12.2020 03:30




Advanced Placement (AP), 07.12.2020 03:30



Arts, 07.12.2020 03:30

History, 07.12.2020 03:30

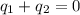
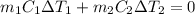
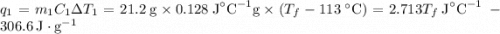
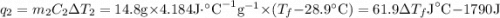
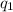 and
and 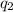 and combinibg like terms, we get
and combinibg like terms, we get![168.0\text{T}_{f}\: ^{\circ}\text{C} ^{-1}\:- 2096 = 0]\\](/tpl/images/0060/4306/42ee8.png)