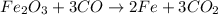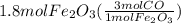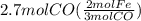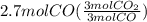Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co, how many moles of each product are formed? 5.4 moles fe and 1.8 moles co2 2.7 moles fe and 0.9 moles co2 3.6 moles fe and 5.4 moles co2 1.8 moles fe and 2.7 moles co2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

You know the right answer?
Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co,...
Questions

Mathematics, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00

Biology, 22.06.2019 09:00





Biology, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00




Biology, 22.06.2019 09:00

Chemistry, 22.06.2019 09:00

History, 22.06.2019 09:00



History, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00

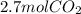 .
.