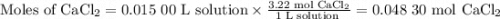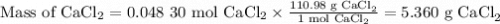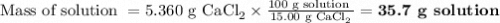Chemistry, 07.07.2019 03:00 staz13wiggins
If you add water to a 15.00 ml solution of 3.22 m cacl2 (mm=110.98 g/mol) in order to create a solution that is 15.00% cacl2 by mass, what is the final mass of the new solution. the density of water is exactly 1.00 g/ml. assume that the density of the cacl2 solution is also exactly 1.00 g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
If you add water to a 15.00 ml solution of 3.22 m cacl2 (mm=110.98 g/mol) in order to create a solut...
Questions



Physics, 28.06.2019 20:00



Biology, 28.06.2019 20:00

Chemistry, 28.06.2019 20:00

Biology, 28.06.2019 20:00



History, 28.06.2019 20:00

Mathematics, 28.06.2019 20:00

Biology, 28.06.2019 20:00




Mathematics, 28.06.2019 20:00

Mathematics, 28.06.2019 20:00