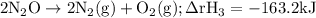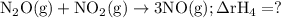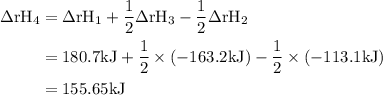Chemistry, 07.07.2019 02:30 villarrealc1987
Given the following data: n2(g)2no(g)++o2(g)o2(g)2n2o(g)→→→2n o(g),2no2(g),2n2(g)+o2(g),δh=+180.7 kjδh=−113.1kjδh=−163.2kj use hess's law to calculate δh for the following reaction: n2o(g)+no2(g)→3no(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:50
Aluminum–lithium (al-li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. a commercial aircraft skin material having a density of 2.47 g/cm3 is desired. compute the concentration of li (in wt%) that is required.
Answers: 3

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2


You know the right answer?
Given the following data: n2(g)2no(g)++o2(g)o2(g)2n2o(g)→→→2n o(g),2no2(g),2n2(g)+o2(g),δh=+180.7 k...
Questions



Mathematics, 24.04.2020 20:43

Spanish, 24.04.2020 20:43



English, 24.04.2020 20:43

Mathematics, 24.04.2020 20:43








Mathematics, 24.04.2020 20:43


History, 24.04.2020 20:43

Mathematics, 24.04.2020 20:43

Mathematics, 24.04.2020 20:43