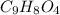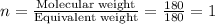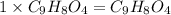Chemistry, 07.07.2019 02:30 jesh0975556
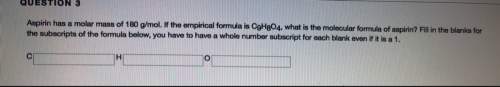
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular formula of aspirin ? fill in the blanks for the subscripts of the formula below. you have to have a whole number subscript for each blank even if it is a 1. c__h__o__

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1



You know the right answer?
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular form...
Questions




Mathematics, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11



Chemistry, 01.04.2020 16:11

English, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11

Medicine, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11







Mathematics, 01.04.2020 16:11