
At 2000 ∘c the equilibrium constant for the reaction 2no(g)⇌n2(g)+o2(g) is kc=2.4×103. you may want to reference (pages 641 - 644) section 15.6 while completing this problem. part a if the initial concentration of no is 0.175 m, what is the equilibrium concentration of no? g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1


Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
At 2000 ∘c the equilibrium constant for the reaction 2no(g)⇌n2(g)+o2(g) is kc=2.4×103. you may want...
Questions

Social Studies, 16.04.2020 22:51



Mathematics, 16.04.2020 22:51

Mathematics, 16.04.2020 22:51








Mathematics, 16.04.2020 22:51


Computers and Technology, 16.04.2020 22:51


Health, 16.04.2020 22:51



Biology, 16.04.2020 22:52

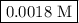 .
.
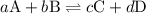
![{K_{\text{c}}}=\dfrac{{{{\left[ {\text{C}} \right]}^c}{{\left[ {\text{D}} \right]}^d}}}{{{{\left[ {\text{A}} \right]}^a}{{\left[ {\text{B}} \right]}^b}}}](/tpl/images/0058/9264/8d53d.png)
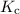 is the equilibrium constant.
is the equilibrium constant.
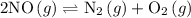
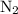 and
and 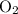 become x at equilibrium.
become x at equilibrium.
![{K_{\text{c}}}=\dfrac{{\left[ {{{\text{N}}_2}} \right]\left[{{{\text{O}}_2}} \right]}}{{{{\left[ {{\text{NO}}} \right]}^2}}}](/tpl/images/0058/9264/f4ed4.png) …… (1)
…… (1)  for
for 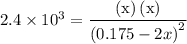 …… (2)
…… (2)
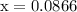
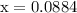
![\begin{aligned}\left[ {{\text{NO}}} \right]&= 0.175 - 2\left( {0.0866} \right)\\&= {\text{ 0}}{\text{.0018 M}}\\\end{aligned}](/tpl/images/0058/9264/23268.png)


