
If 23.7 g of al(oh)3(s) are mixed with 29.5 g of h2so4(s) and the reaction is run, answer the following questions: (a) what is the limiting reagent? (b) assuming no side reactions, how much h2o(l) can be produced under these conditions? (c) if 2.21 g of h2o(g) are produced in the lab under these conditions, what is the percent yield? (d) how many grams of the reactant in excess remains at the end of the experiment? data: atomic mass al = 26.98, h = 1.008, o = 16.00, s = 32.07,

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
If 23.7 g of al(oh)3(s) are mixed with 29.5 g of h2so4(s) and the reaction is run, answer the follow...
Questions


Mathematics, 21.05.2021 14:00

English, 21.05.2021 14:00



Mathematics, 21.05.2021 14:00


Mathematics, 21.05.2021 14:00

English, 21.05.2021 14:00

Mathematics, 21.05.2021 14:00



English, 21.05.2021 14:00

Arts, 21.05.2021 14:00

French, 21.05.2021 14:00


Mathematics, 21.05.2021 14:00

Mathematics, 21.05.2021 14:00


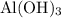 can behave as a base and neutralize sulfuric acid
can behave as a base and neutralize sulfuric acid 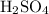 as in the following equation:
as in the following equation: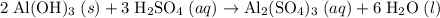 (Balanced)
(Balanced)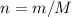 . Thus the ratio between the number of moles of the two reactants available:
. Thus the ratio between the number of moles of the two reactants available:![n(\text{Al}(\text{OH})_3, \text{supplied}) / n(\text{H}_2\text{SO}_4, \text{supplied})\\= [m(\text{Al}(\text{OH})_3)/ M(\text{Al}(\text{OH})_3)] / [n(\text{H}_2\text{SO}_4) / M(\text{H}_2\text{SO}_4)]\\= [23.7 / (26.98 + 3 \times(16.00 + 1.008))]/[29.5 / (2 \times 1.008 + 32.07 + 4 \times 16.00)]\\\approx 1.01](/tpl/images/0058/7818/2e46d.png)
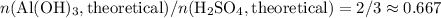
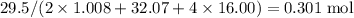 of sulfuric acid is supplied in this reaction as the limiting reagent.
of sulfuric acid is supplied in this reaction as the limiting reagent.  moles of water molecules are produced for every
moles of water molecules are produced for every  moles of sulfuric acid consumed. The reaction would thus give rise to
moles of sulfuric acid consumed. The reaction would thus give rise to 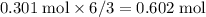 of water molecules, which have a mass of
of water molecules, which have a mass of 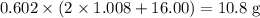 .
.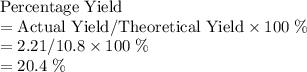
 moles of aluminum hydroxide
moles of aluminum hydroxide 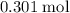 of sulfuric acid is initially available as previously stated such that
of sulfuric acid is initially available as previously stated such that 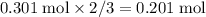 , or
, or 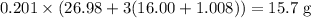 , of
, of 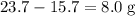 of
of 

