
What does the pauli exclusion principle state?
a) electrons will be added to an atom at the lowest possible level or sublevel.
b) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.
c) an orbital can contain two electrons only if all other orbitals at that sublevel contain at least one electron.
d) two electrons occupy the same orbital only if they have opposite spins.
e) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
What does the pauli exclusion principle state?
a) electrons will be added to an atom a...
a) electrons will be added to an atom a...
Questions



Mathematics, 31.03.2021 05:00

English, 31.03.2021 05:00

Mathematics, 31.03.2021 05:00


Mathematics, 31.03.2021 05:00


Spanish, 31.03.2021 05:00

Mathematics, 31.03.2021 05:00


Mathematics, 31.03.2021 05:00

Mathematics, 31.03.2021 05:00



Mathematics, 31.03.2021 05:00

Social Studies, 31.03.2021 05:00


Mathematics, 31.03.2021 05:00

English, 31.03.2021 05:00

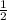 or
or 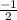 .
.

