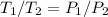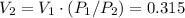Chemistry, 06.07.2019 17:00 miguel454545
Explain how you can use boyle's law to determine the new volume of gas when it's pressure is increased from 170 to 5: 40 the original volume of gas is one leader assume the temperature and number of particles are consistent what is the new volume

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2


You know the right answer?
Explain how you can use boyle's law to determine the new volume of gas when it's pressure is increas...
Questions






Mathematics, 28.06.2019 06:00

History, 28.06.2019 06:00

History, 28.06.2019 06:00

History, 28.06.2019 06:00





Business, 28.06.2019 06:00

Mathematics, 28.06.2019 06:00

English, 28.06.2019 06:00




 Initial Pressure
Initial Pressure  Final Pressure
Final Pressure