If 5 moles of p4 reacted with 22 moles cl2 according to the above reaction, determine:
a. how...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Questions

Physics, 25.10.2021 01:00

English, 25.10.2021 01:00

History, 25.10.2021 01:00

History, 25.10.2021 01:00

Mathematics, 25.10.2021 01:00



English, 25.10.2021 01:00

Social Studies, 25.10.2021 01:00




Mathematics, 25.10.2021 01:00




Physics, 25.10.2021 01:00

Chemistry, 25.10.2021 01:00

English, 25.10.2021 01:00

English, 25.10.2021 01:00

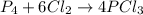
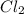 gives 4 mole of
gives 4 mole of 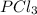 , then 22 moles of
, then 22 moles of 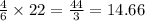 moles of
moles of 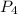 , then 22 moles of
, then 22 moles of 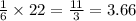 moles of
moles of 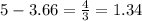 moles.
moles.

