
Chemistry, 06.07.2019 13:00 briannagotfanz
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-gram sample undergoes decomposition, producing 283.4 grams of carbon, 31.7 grams of hydrogen, and 377.4 grams of oxygen. what is the molecular formula of the compound?

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-g...
Questions











Mathematics, 16.03.2020 22:33

History, 16.03.2020 22:33

History, 16.03.2020 22:33







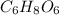 .
.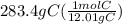
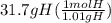
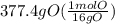
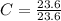 = 1
= 1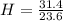 = 1.33
= 1.33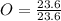 = 1
= 1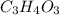 .
.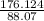 = 2
= 2


