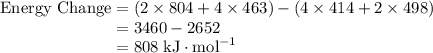Chemistry, 06.07.2019 12:30 sillyvanna
Calculate the heat of the reaction using average bond dissociation energies ch₄ + 2o₂ ⇒ co₂ + 2h₂o i really don't understand this college chemistry question. it's due tonight! you to anyone who can explain and answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Calculate the heat of the reaction using average bond dissociation energies ch₄ + 2o₂ ⇒ co₂ + 2h₂o...
Questions

History, 28.07.2019 10:00

Mathematics, 28.07.2019 10:00




Biology, 28.07.2019 10:00

Computers and Technology, 28.07.2019 10:00


Arts, 28.07.2019 10:00


English, 28.07.2019 10:00

History, 28.07.2019 10:00





History, 28.07.2019 10:00

World Languages, 28.07.2019 10:00


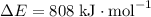
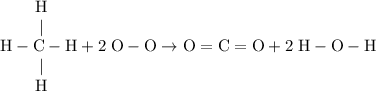
 as in one methane
as in one methane 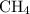 molecule;Two oxygen-oxygen bonds
molecule;Two oxygen-oxygen bonds 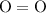 as in two oxygen
as in two oxygen 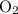 molecules with one bond in each molecule.
molecules with one bond in each molecule. 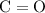 as in one carbon dioxide
as in one carbon dioxide 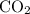 molecule.Four hydrogen-oxygen single bonds
molecule.Four hydrogen-oxygen single bonds 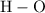 as in two water
as in two water 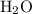 molecules.
molecules.![\text{Energy Change} = \text{Energy Released} - \text{Energy Absorbed}\\\phantom{\text{Energy Change}} = E[\text{Bonds Formed}] - E[\text{Bonds Broken}]](/tpl/images/0057/8855/53ce1.png)
![E[\text{C}-\text{H}] = 414 \; \text{kJ} \cdot \text{mol}^{-1}](/tpl/images/0057/8855/09656.png) ;
;![E[\text{O}=\text{O}] =498 \; \text{kJ} \cdot \text{mol}^{-1}](/tpl/images/0057/8855/33ae3.png) ;
;![E[\text{C}=\text{O}] =804 \; \text{kJ} \cdot \text{mol}^{-1}](/tpl/images/0057/8855/7cfd6.png) ;
;![E[\text{H}-\text{O}] =463 \; \text{kJ} \cdot \text{mol}^{-1}](/tpl/images/0057/8855/b9814.png) .
.