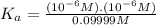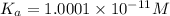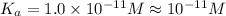Chemistry, 06.07.2019 07:00 shaheedbrown06
The ph of 0.10 m solution of an acid is 6. what is the acid dissociation constant of the acid? 10-11 10-12 10-5 10-6

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
The ph of 0.10 m solution of an acid is 6. what is the acid dissociation constant of the acid? 10-...
Questions

Mathematics, 07.05.2020 09:00




History, 07.05.2020 09:00

History, 07.05.2020 09:00

Geography, 07.05.2020 09:00

Mathematics, 07.05.2020 09:00



Computers and Technology, 07.05.2020 09:00

Mathematics, 07.05.2020 09:00


Social Studies, 07.05.2020 09:00

English, 07.05.2020 09:00




Mathematics, 07.05.2020 09:00

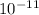 .
.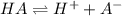
![pH=-log([H^+])](/tpl/images/0057/0083/79766.png)
![[H^+]=antilog(-pH)](/tpl/images/0057/0083/3f393.png)
![[H^+]=antilog(-6)=10^{-6}M](/tpl/images/0057/0083/e47e5.png)
![[H^+]=[A^-]=10^{-6}M](/tpl/images/0057/0083/e783b.png)
![[HA]=[HA]-[H^+]](/tpl/images/0057/0083/07373.png)
![[HA]=0.1M-10^{-6}M](/tpl/images/0057/0083/2f8e8.png)
![[HA]=0.09999M](/tpl/images/0057/0083/be68b.png)
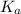 is given as:
is given as:![K_a=\frac{[A^-][H^+]}{HA}](/tpl/images/0057/0083/a617d.png)
![[H^+],[A^-]\text{ and }[HA]](/tpl/images/0057/0083/ad98d.png) in the above equation, we get
in the above equation, we get