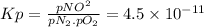Chemistry, 06.07.2019 06:30 NateTheBeast12
The equilibrium constant (kp) for the formation of the air pollutant nitric oxide (no) in an automobile engine at 537°c is 4.5 × 10−11. n2(g) + o2(g) ⇌ 2no(g) (a) calculate the partial pressure of no under these conditions if the partial pressures of nitrogen and oxygen are 3.00 and 0.012 atm, respectively.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
The equilibrium constant (kp) for the formation of the air pollutant nitric oxide (no) in an automob...
Questions



Mathematics, 14.04.2021 23:50



English, 14.04.2021 23:50


Mathematics, 14.04.2021 23:50





Mathematics, 14.04.2021 23:50

Mathematics, 14.04.2021 23:50

Mathematics, 15.04.2021 01:00



Spanish, 15.04.2021 01:00