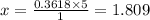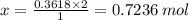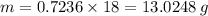Chemistry, 06.07.2019 06:30 jscout2468
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are allowed to react with 1.818 moles of o2, and this is the only reaction which occurs, what is the maximum mass of water that could be produced?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1


Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are a...
Questions

History, 13.12.2019 23:31

Mathematics, 13.12.2019 23:31

Computers and Technology, 13.12.2019 23:31



Biology, 13.12.2019 23:31


Mathematics, 13.12.2019 23:31







Health, 13.12.2019 23:31



Mathematics, 13.12.2019 23:31