
Chemistry, 06.07.2019 05:30 crawford184232323234
Asample of an unknown compound contains 82.63% c, and 17.37% h. determine the empirical formula.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Asample of an unknown compound contains 82.63% c, and 17.37% h. determine the empirical formula....
Questions




Health, 13.04.2020 02:12

Mathematics, 13.04.2020 02:12

Mathematics, 13.04.2020 02:12

English, 13.04.2020 02:12



Biology, 13.04.2020 02:12

Mathematics, 13.04.2020 02:12

Spanish, 13.04.2020 02:13

Mathematics, 13.04.2020 02:13


Mathematics, 13.04.2020 02:13

Mathematics, 13.04.2020 02:13

Mathematics, 13.04.2020 02:13


Mathematics, 13.04.2020 02:13

Engineering, 13.04.2020 02:13

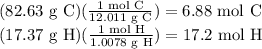
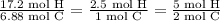
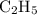 .
.

