
Several solutions of nickel (ii) sulfate ranging from 1.00m to 0.200 m are exposed to light in a colorimeter and their absorbance is recorded in a beer-lambert law experiment. why does a solution of nickel(ii) sulfate represent a suitable solution to be studied in this experiment, whereas similar solutions of sodium chloride do not?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2


Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2
You know the right answer?
Several solutions of nickel (ii) sulfate ranging from 1.00m to 0.200 m are exposed to light in a col...
Questions


Mathematics, 24.09.2019 21:30



Mathematics, 24.09.2019 21:30


Health, 24.09.2019 21:30


Social Studies, 24.09.2019 21:30


Mathematics, 24.09.2019 21:30


Computers and Technology, 24.09.2019 21:30

Mathematics, 24.09.2019 21:30






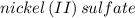 is green in color but a solution of sodium chloride is colorless that is why
is green in color but a solution of sodium chloride is colorless that is why 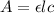
 = molar absorptivity
= molar absorptivity

