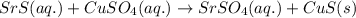Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Aqueous strontium sulfide and aqueous copper(ii) sulfate predict the products of each of these reac...
Questions


History, 24.03.2021 07:10

English, 24.03.2021 07:10

Mathematics, 24.03.2021 07:10

Computers and Technology, 24.03.2021 07:10


Mathematics, 24.03.2021 07:10


History, 24.03.2021 07:10






Mathematics, 24.03.2021 07:10


Biology, 24.03.2021 07:10


Mathematics, 24.03.2021 07:10

Mathematics, 24.03.2021 07:10