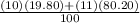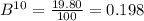Chemistry, 06.07.2019 04:30 codycollier
Calculate the average atomic mass of b. the isotopes and abundances are 10b, 19.80% and 11b, 80.20%. round answer to 3 significant digits. a. 1.98 amu b. 10.8 amu c. 10 amu d. 8.82 amu

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Calculate the average atomic mass of b. the isotopes and abundances are 10b, 19.80% and 11b, 80.20%....
Questions


English, 10.02.2022 03:10

Chemistry, 10.02.2022 03:20

History, 10.02.2022 03:20



English, 10.02.2022 03:20

Social Studies, 10.02.2022 03:20


Mathematics, 10.02.2022 03:20

English, 10.02.2022 03:20

English, 10.02.2022 03:20

Mathematics, 10.02.2022 03:20


Mathematics, 10.02.2022 03:20

English, 10.02.2022 03:20

Health, 10.02.2022 03:20


Biology, 10.02.2022 03:20

Mathematics, 10.02.2022 03:20