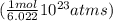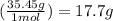Chemistry, 06.07.2019 02:00 unicornpoop54
Determine the mass in grams of the following: a. 2.00 mol n2 b. 3.01 x 1023 atoms cl

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
Determine the mass in grams of the following: a. 2.00 mol n2 b. 3.01 x 1023 atoms cl...
Questions





History, 02.11.2020 16:30



Advanced Placement (AP), 02.11.2020 16:30

History, 02.11.2020 16:30


Geography, 02.11.2020 16:30


Physics, 02.11.2020 16:30




English, 02.11.2020 16:30

Social Studies, 02.11.2020 16:30


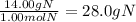
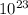 atms
atms