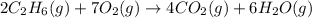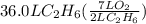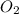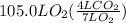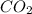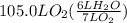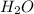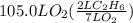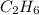Chemistry, 05.07.2019 22:00 aidendespatieshakim
How many liters of gas will be in the closed reaction flask when 36.0l of ethane (c2h6) is allowed to react with 105.0l of oxygen (under constant pressure and temperature) to form carbon dioxide gas and water vapor? assume ideal gas behavior.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
How many liters of gas will be in the closed reaction flask when 36.0l of ethane (c2h6) is allowed t...
Questions





Computers and Technology, 06.11.2019 04:31


Mathematics, 06.11.2019 04:31




Physics, 06.11.2019 04:31

Biology, 06.11.2019 04:31

Health, 06.11.2019 04:31

Mathematics, 06.11.2019 04:31




Biology, 06.11.2019 04:31

Biology, 06.11.2019 04:31

Chemistry, 06.11.2019 04:31