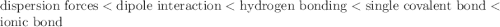Chemistry, 05.07.2019 18:30 jaylan11brown
Place the following types of attraction weakest to strongest: dispersion ionic bond single covalent bond hydrogen bond dipole interaction

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2


Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

You know the right answer?
Place the following types of attraction weakest to strongest: dispersion ionic bond single covalen...
Questions

English, 06.05.2020 08:44



Mathematics, 06.05.2020 08:44




Mathematics, 06.05.2020 08:44

Social Studies, 06.05.2020 08:44


Mathematics, 06.05.2020 08:44

Mathematics, 06.05.2020 08:44


Mathematics, 06.05.2020 08:44

Mathematics, 06.05.2020 08:44


Mathematics, 06.05.2020 08:44

Mathematics, 06.05.2020 08:44

Mathematics, 06.05.2020 08:44

Mathematics, 06.05.2020 08:44