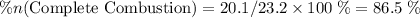Chemistry, 05.07.2019 18:00 amortalstardev
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incomplete combustion produces h2o and co, which not only reduces the efficiency of the engine using the fuel but is also toxic. in a certain test run, 1.000 gallon (gal) of octane is burned in an engine. the total mass of co, co2, and h2o produced is 11.53 kg. calculate the efficiency of the process; that is, calculate the fraction of octane converted to co2. the density of octane is 2.650 kg/gal.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 11:40
Which of the following observations indicates that an atom has neutrons? some uncharged particles are scattered by a beryllium atom when it hits a gold foil. some uncharged particles bounce back from a gold foil when it is bombarded with alpha particles. a radiation consisting of uncharged particles is emitted when alpha particles strike beryllium atoms. a radiation which attracts electrons is produced when a beryllium atom is bombarded with alpha particles.
Answers: 2
You know the right answer?
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incompl...
Questions



Computers and Technology, 29.08.2019 02:10

Mathematics, 29.08.2019 02:10





English, 29.08.2019 02:10






Mathematics, 29.08.2019 02:10



Mathematics, 29.08.2019 02:10


Physics, 29.08.2019 02:10

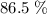 of octane had been converted to carbon dioxide CO₂.
of octane had been converted to carbon dioxide CO₂.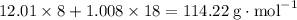
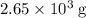 , which corresponds to
, which corresponds to 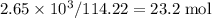 of octane.
of octane.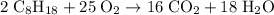
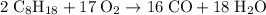
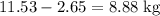 heavier than that of the octane supplied. Thus
heavier than that of the octane supplied. Thus 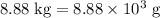 of oxygen were consumed in the combustion. There are
of oxygen were consumed in the combustion. There are 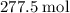 of oxygen molecules in
of oxygen molecules in 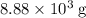 of oxygen.
of oxygen. (
(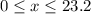 ). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal
). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal 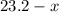 .
.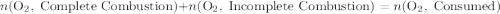
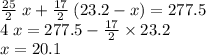
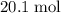 out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.
out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.